Table of Contents
At the center of every atom is a nucleus. Breaking that nucleus apart—or combining two nuclei together—can release large amounts of energy. Nuclear weapons use that energy to create an explosion.
Fission and fusion
All matter is composed of atoms: incredibly small structures that house different combinations of three particles, known as protons, neutrons, and electrons.
At the center of each atom is a “nucleus” (the plural of which is “nuclei”), where neutrons and protons are bound in close proximity together. Most nuclei are relatively stable, meaning the makeup of their neutrons and protons is comparatively static and unchanging.
During fission, the nuclei of certain heavy atoms split into smaller, lighter nuclei, releasing excess energy in the process. This can sometimes occur spontaneously, but can also, in certain nuclei, be induced from outside. A neutron is shot at the nucleus and is absorbed, causing instability and fission. In some elements—such as certain isotopes of uranium and plutonium—the fission process also releases excess neutrons, which can trigger a chain reaction if they’re absorbed by nearby atoms.
Fusion works in reverse: when exposed to extremely high temperatures and pressures, some lightweight nuclei can fuse together to form heavier nuclei, releasing energy in the process.
In modern nuclear weapons, which use both fission and fusion, a single warhead can release more explosive energy in a fraction of a second than all of the weapons used during World War II combined—including Fat Man and Little Boy, the two atom bombs dropped on Japan.
How they work
All nuclear weapons use fission to generate an explosion. “Little Boy”—the first nuclear weapon ever used during wartime—worked by shooting a hollow uranium-235 cylinder at a target “plug” of the same material.
Nuclear fuel
Only certain isotopes of certain elements can undergo fission (an isotope is a variation of the same element with different numbers of neutrons in the nucleus). Plutonium-239 and uranium-235 are the most common isotopes used in nuclear weapons.
Each piece by itself was not enough to constitute a critical mass (the minimum amount of nuclear material needed to maintain fission)—but by colliding the pieces, critical mass was reached and a fission chain reaction occurred.
Modern nuclear weapons work slightly differently. Critical mass depends on the density of the material: as the density increases, the critical mass decreases. Instead of colliding two sub-critical pieces of nuclear fuel, modern weapons detonate chemical explosives around a sub-critical sphere (or “pit”) of uranium-235 or plutonium-239 metal. The force from the blast is directed inward, compressing the pit and bringing its atoms closer together. Once dense enough to reach the critical mass, neutrons are injected, initiating a fission chain reaction and producing an atomic explosion.
In fusion weapons (also called “thermonuclear” or “hydrogen” weapons), the energy from an initial fission explosion is used to “fuse” hydrogen isotopes together. The energy released by the weapon creates a fireball that reaches several tens of million degrees—temperatures in the same range as the center of the sun (which also runs on fusion).
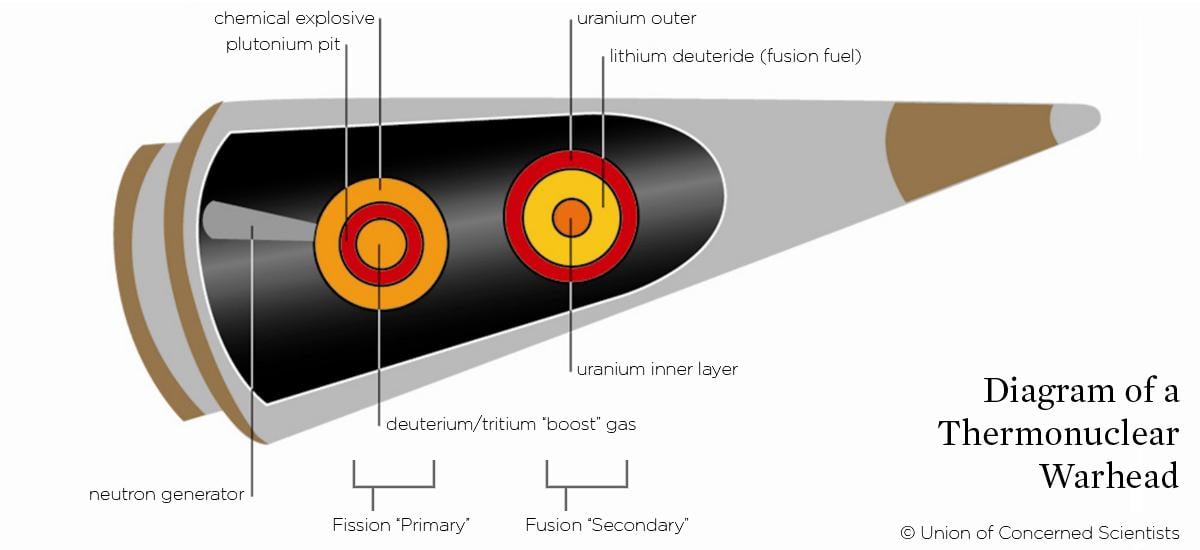
Warheads in-depth
The explosions used in thermonuclear weapons are often described as a primary (the chemical and fission explosions) and secondary (the subsequent fusion blast). However, the actual mechanisms are considerably more complicated.
For example, a pure fission primary is inefficient—the plutonium pit will blow itself apart before most of the plutonium-239 can fission. Instead, the reaction can be “boosted” by including hydrogen gas (consisting of the isotopes deuterium and tritium) in the center of a hollow pit. As the surrounding plutonium fissions, the hydrogen gas undergoes fusion and releases neutrons, inducing additional fission.
Similarly, the secondary doesn’t consist purely of fusion fuel; layered within it is a fission "spark plug," consisting of either plutonium-239 or uranium-235. As the primary explosion compresses the fuel from the outside, the spark plug material becomes supercritical and fissions, heating the hydrogen from the inside and facilitating further fusion reactions.
Fusion releases neutrons. These neutrons hit a layer of uranium surrounding the fusion fuel causing atoms in it to fission; this fissioning generally contributes more than half of the weapon’s total explosive yield.
Thermonuclear weapons that don’t include this uranium “blanket” are called neutron bombs, as the neutrons freed by fusion are released from the weapon. Neutron bombs therefore create a larger amount of radiation than a normal weapon of the same yield. During the Cold War such weapons were considered for use against tank attacks, with the goal of disabling tank crews without having to physically destroy the tank.

Nuclear fuel
While a number of elements are fissionable (meaning they can undergo fission), only a few are used in nuclear weapons. Most common are the isotopes uranium-235 and plutonium-239 (reminder: isotopes are atoms of the same element that differ only in their number of neutrons).
Uranium is found throughout the world and can be mined from mineral deposits (it can also be extracted from seawater, but doing so is currently much more expensive). However, only a small fraction (less than one percent) of naturally occurring uranium is uranium-235. Producing usable uranium requires a process of “enrichment,” in which different uranium isotopes are separated and concentrated (usually using centrifuges, which operate like salad spinners). This is extremely costly, difficult, and time-consuming, and is one of the central barriers to constructing a nuclear bomb.
Plutonium can also be used, but only occurs naturally in trace amounts. It can, however, be produced as a fission byproduct in nuclear reactors, then separated by a process called “reprocessing.” Plutonium separation is easier than uranium enrichment—it involves separating different elements, not different isotopes of the same element—but it’s a highly radioactive process that requires heavily shielded facilities with remote-handling equipment.
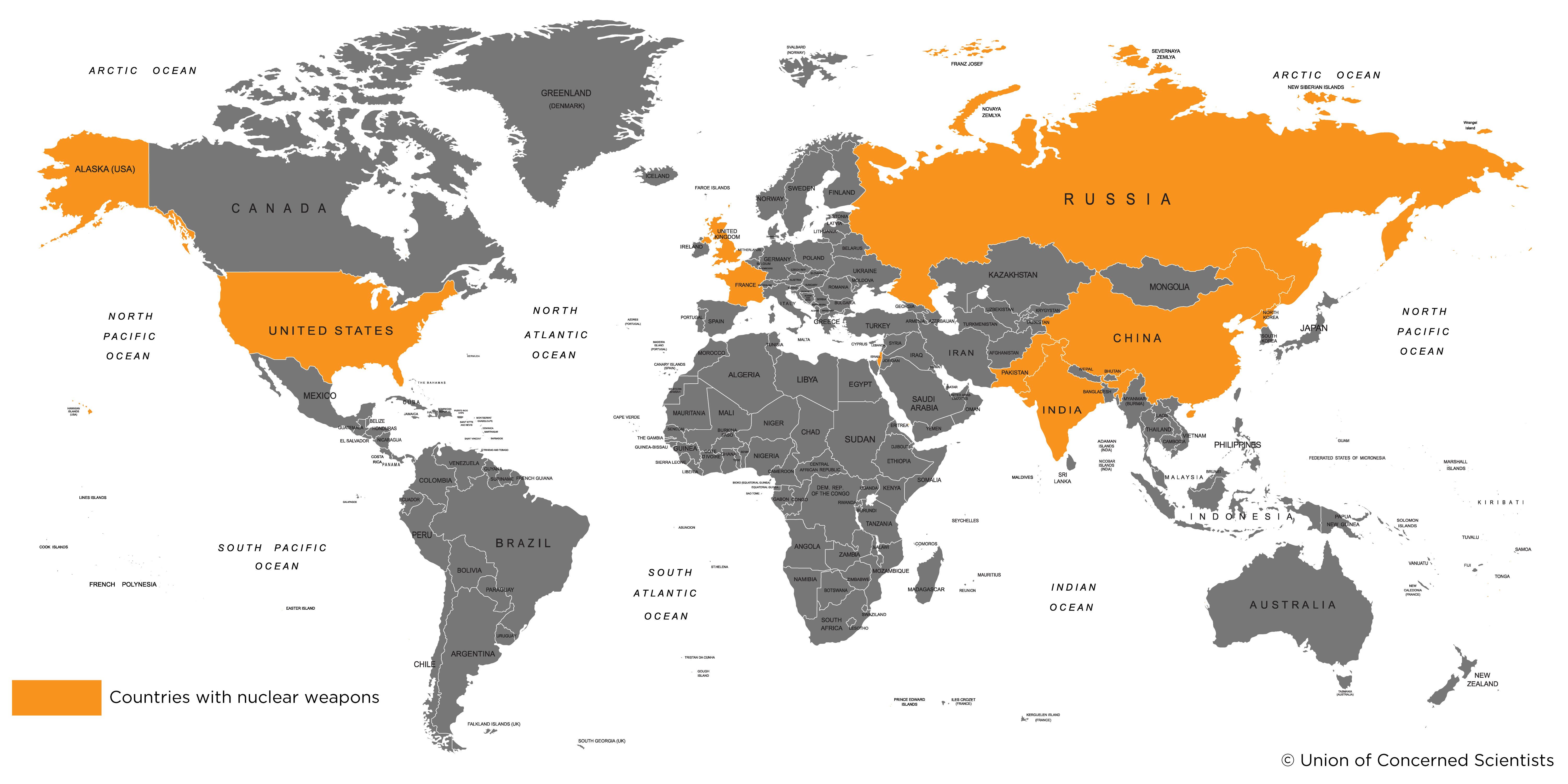
Who has nuclear weapons?
The United States was the first country to develop nuclear weapons, detonating the first fission device in 1945. Seven years later the United States successfully tested the first hydrogen bomb during “Operation Ivy” (physicist Richard Garwin helped build that device, and today serves on the board of the Union of Concerned Scientists). As of 2018, the United States had an estimated 6,500 nuclear warheads, including retired (awaiting dismantlement), stored, and deployed weapons.
The Soviet Union first developed nuclear capabilities in 1949. Russia’s modern day arsenal includes an estimated 7,000 warheads.
France (~300 warheads), China (~260), the United Kingdom (~215), Pakistan (~130), and India (~120) also have nuclear weapons. Israel has not officially acknowledged its nuclear capabilities. Estimates of its arsenal have typically been around 80 warheads, although some estimates are significantly larger.
North Korea’s capabilities are largely unknown. It’s suspected it may have a limited arsenal of 5-10 weapons, but may have material to build twice that many.
What can I do?
At the peak of the Cold War in the mid-1980’s, the world had a combined total of over 60,000 nuclear warheads. Today that number is closer to 15,000, representing a 75 percent reduction.
This progress was made possible thanks to leaders and advocates who recognized the very real dangers of nuclear war. Even a limited nuclear exchange could kill millions of people, cause severe climate effects, and wreak havoc on the world’s political and economic structures; a full-blown nuclear war would do irreparable harm to the world as we know it.
More work remains to be done, including further cuts, faster dismantlement, and de-alerting (hundreds of U.S. missiles are still kept on hair-trigger alert, increasing the risk of an accidental, unauthorized, or mistaken launch).
You can help. Visit our action center to get involved.




